
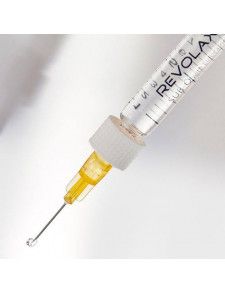
25Gx40 Flexible...
115,83 € tax excl.





43,33 €
Across Revolax
Injectable hyaluronic acid
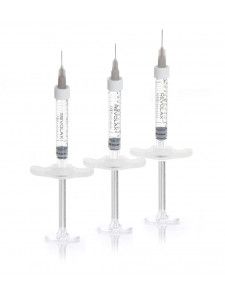
1 syringe of 1,1mL

 100% secured payment
100% secured payment
All of your payments are secured. A doubt? Feel free to contact Us!
 Delivery
Delivery
Shipping on the day of your order, delivery with DHL.
 Quick to respond customer service
Quick to respond customer service
Via phone, Whatsapp, text, e-mail.Feel free to ask all of your questions to our team of pharmacists
Due to its advanced ability to shape, maintain its structure and longevity, it is recommended for the treatment of deep to severe wrinkles, including nasolabial and facial contours (cheek, chin or nose).
Hyaluronic Acid: 24 mg / ml
HCl Lidocaine 0,3%
Cross-linking agent: BDDE
Volume 1.1ml
12 to 18 months
Across is a Korean laboratory.
It is CE marked.
REVOLAX:
- High purity hyaluronic acid non-animal
- Natural substances extracted from bacteria
- High purity (endotoxin <0,0015 UI / mg)
- Biodegradable (natural absorption)
- High cross-linking: Across fine cross-linking technology
- Improving durability
- Highly coherent single-phase
- Regular and dense pattern
- Stable and constant gel structure
- Natural effect / Soft injection
- High viscosity and elasticity
- Intensify the persistence of longer durability
- Maintains elasticity by tightly supporting the skin tissue
REVOLAX SUB-Q lidocaine is a sterile, pyrogen-free, physiological gel of cross-linked hyaluronic acid of non-animal origin with the addition of 0.3% lidocaine hydrochloride. It is a colourless, unscented and highly viscous aqueous gel.
REVOLAX SUB-Q lidocaine is provided as a sterile volume of 1.1 ml in a single-use glass syringe with two sterilized needles.
REVOLAX SUB-Q Lidocaine is recommended for intradermal implantation for volume loss and morphological asymmetry of the face. The addition of lidocaine provides an analgesic effect during treatment.
· Do not inject REVOLAX SUB-Q Lidocaine into the eye area (eye circle or eyelids).
· Do not inject REVOLAX SUB-Q Lidocaine into the blood vessels (intravascular).
· REVOLAX SUB-Q Lidocaine should not be used in:
- Pregnant or nursing women
- Persons under 18 years of age
- Patients known to be hypersensitive to hyaluronic acid.
- Patients who tend to develop hypertrophic scars.
· REVOLAX SUB-Q Lidocaine should not be used in combination with laser therapy, chemical peeling or skin abrasion.
· REVOLAX SUB-Q Lidocaine should not be used in areas with inflammatory and infectious skin problems.
- The total dose of lidocaine administered should be considered if a dental block or topical administration of lidocaine are used simultaneously. High doses of lidocaine (more than 400 mg) may cause acute toxic reactions with symptoms affecting the central nervous system and cardiac conduction.
· Lidocaine should be used with caution in patients receiving other local anaesthetics or structural agents such as some anti-arrhythmics, as systemic toxicity may be additive.
· Lidocaine should be used with caution in patients with epilepsy, altered cardiac conduction, severe alteration of liver function or severe renal dysfunction. Peribulbar injections of local anaesthetics carry a small risk of persistent ocular muscle dysfunction.
REVOLAX SUBQ lidocaine is intended for use as an intradermal implant only.
Ensure that the product has not expired, and that sterility has not been compromised before use. The product is for single use only; do not reuse.
If reused, this may lead to reduced performance of the device and serious cross-infection. Used needles and syringes must be thrown away in a designated container.
REVOLAX SUBQ lidocaine is packaged for single patient use. Do not re-sterilise. Do not use if the packaging is open or damaged.
· REVOLAX SUBQ Lidocaine should not be injected into an area where there is an implant.
· REVOLAX SUBQ Lidocaine should not be mixed with other products prior to implantation.
· Hyaluronic acid products have a known incompatibility with quaternary ammonium salts such as benzalkonium chloride.
· Do not use in patients with coagulation disorders or in patients who have been treated with thrombolytic anticoagulants or platelet aggregation inhibitors within the previous 2 weeks. Also, it is recommended to avoid taking aspirin, non-steroidal anti-inflammatory drugs or high doses of vitamin C the week before the injection.
· Patients are advised not to wear make-up for 12 hours after the injection and to avoid extended exposure to sunlight, UV light and extreme cold and heat for 2 weeks after the injection.
· If the needle is stuck, do not increase the pressure on the piston rod but stop the injection and replace the needle.
Doctors should inform patients that there are potential adverse reactions that may occur immediately or be delayed after injection. These undesirable effects include, but are not limited to, the following:
· Inflammatory reactions such as redness, swelling and sensitivity may occur at the injection location. These reactions may last for two weeks.
· Nodules or indurations may also occur at the injection spot.
· Bruises
· Coloration or discoloration of the injection site.
· Poor or weak filling effect.
· Glabellar necrosis, abscess formation, granuloma and immediate or delayed hypersensitivity have been reported in the literature following hyaluronic administration and injection. It is therefore important to consider these possible complications.
Patients should report to their doctor as soon as possible any inflammatory reactions that persist for more than a week or any other side effects that develop. The doctor should treat these reactions properly.
Any other undesirable side effects associated with REVOLAX SUB-Q lidocaine injection should be reported to the distributor and/or manufacturer.
Compatible HyaluronPen ( pour cette utilisation vous devez avoir suivi et réussi une formation certifiée et agrée à ces techniques. En aucun cas vous ne devez essayer d'injecter le produit sans la formation adéquate sous peine d’entraîner des blessures graves. French Filler n'accepte aucune responsabilité en cas d'utilisation en dehors de ces termes et conditions.)
Anonymous A. published the 27/01/2023 following an order made on 12/01/2023
.........................................
Anonymous A. published the 23/12/2022 following an order made on 19/12/2022
👍👍👍👍👍
Anonymous A. published the 23/11/2022 following an order made on 21/11/2022
👍👍👍👍👍👍extra
Anonymous A. published the 15/10/2022 following an order made on 10/10/2022
Super produit
Anonymous A. published the 02/10/2022 following an order made on 28/09/2022
Super
Anonymous A. published the 21/07/2022 following an order made on 12/07/2022
👍👍👍👍👍👍
Anonymous A. published the 04/04/2022 following an order made on 06/02/2022
Super Produit bien décrit
Anonymous A. published the 15/10/2021 following an order made on 16/09/2021
👍👍👍👍👍
Anonymous A. published the 14/10/2021 following an order made on 04/10/2021
Très bon produit comble bien